Enter the total number of atoms of a substance and the average atomic mass of the substance into the calculator. The calculator will convert the total atoms to grams.
Atoms to Grams Formula
The SI unit of mass is a kilogram, which is defined by taking the fixed numerical value of the Planck constant h to be 6.626 070 15 × 10⁻³⁴ when expressed in the unit J s, which is equal to kg m² s⁻¹, where the meter and the second are defined in terms of c and Δν Cs.Multiples of kilogram are also commonly used, such as a gram (1/1000 of a kilogram) and a tonne.
- Moles to Atoms Formula. The following formula is used to convert the total moles to total atoms. A = M. 6.0221415.10^23. Where A is the number of atoms; M is the number of moles; Moles to Atoms Definition. Converting moles to atoms is as simple as multiplying the number of moles by the 6.022. 10^23 because by definition that is what a mole.
- How to Convert Atomic Mass Unit to Gram. 1 u = 1.6605402E-24 g 1 g = 6.752E+23 u. Example: convert 15 u to g: 15 u = 15 × 1.6605402E-24 g = 2.4908103E-23 g. Popular Weight And Mass Unit Conversions.
The following equation is used to calculate the total number of grams from atoms.
G = A * AAM
- Where G is grams
- A is the total number of atoms
- AAM is the average atomic mass of the atoms
Atoms to Grams Definition
Converting atoms to grams involves multiply the total number of atoms by the average atomic mass of the atoms.
How to convert atoms to grams?
how to calculate atoms to grams
- First, determine the total number of atoms in the substance
Calculate the total number of atoms your are measuring.
- Next, calculate the AAM
Calculate the average atomic mass of the substance using the calculator linked above or a formula.
- Finally, calculate the grams
Multiply the total atoms by the average atomic mass per atom to calculate the grams.
FAQ
What is average atomic mass?Average atomic mass is the sum of the fractional percents of each atom in a substance.
What is an atom?An atom is a microscopic basic element that makes of everything in the world.
The last step we are going to address in this particular conversion map is the conversion to atoms. Converting between atoms and molecules is much easier than the steps that we have done before. However, it seems to many students because of that they get thrown off and have a difficult time. This conversion is all about how many of each element are in a particular substance. Check out the examples below for a more detailed explanation.
If we wanted to say how many atoms of Br are in NBr3 then we would say 3. Now the only difference with these conversions is we have to turn that statement into a ratio between the molecule (NBr3) and the atom (Br).
| 1 NBr3 |
| 3 Br |
or
| 3 Br |
| 1 NBr3 |
We can also do this for molecules that have only one atom of one element. It just looks kind of silly so most teachers and books will not show it to you. Lets say we had the molecule Ni. How many atoms of Ni are in the molecule of Ni? 1 atom of Ni for every 1 molecule of Ni.
| 1 Ni |
| 1 Ni |
or
| 1 Ni |
| 1 Ni |
Now the above may look trivial and painfully obvious but believe it or not a lot of students run into problems with the above ratio and it is nothing to be ashamed of. It is hard for people to describe and therefore it is hard for students to understand correctly. In these special cases where there is only 1 atom per 1 molecule then you don’t really need to do any extra math steps. However, I will show you to them in my demonstrated examples to make sure everyone understands what is going on.
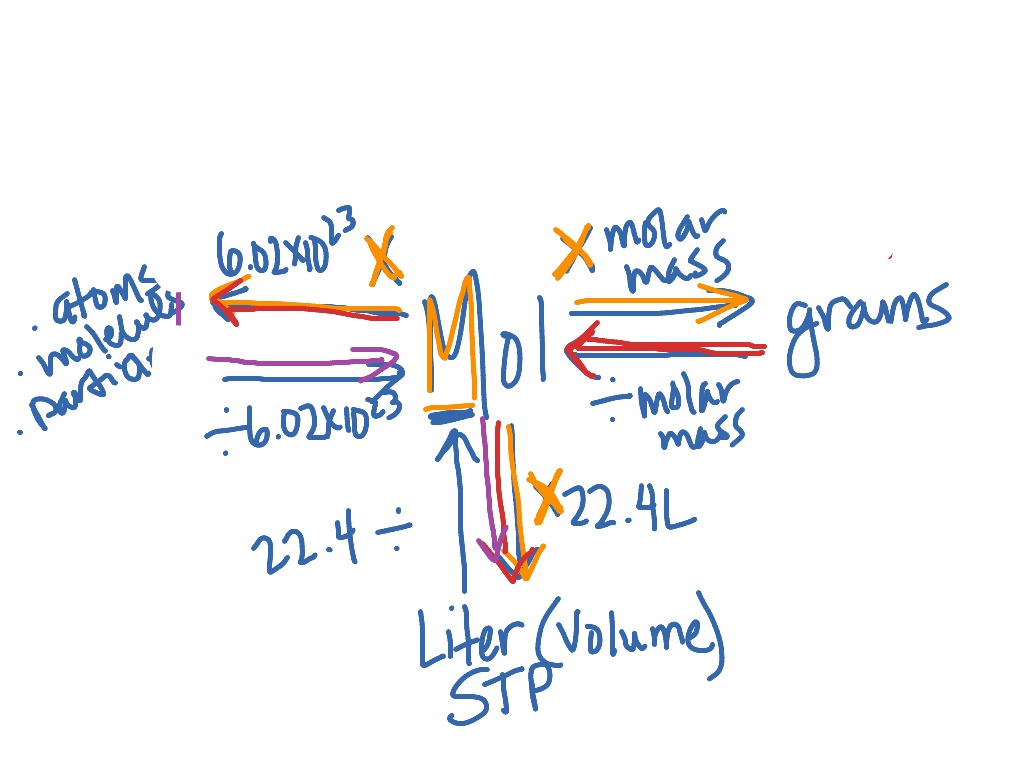
This last step in our conversion map is the arrow furthest to the right (the purple text).
VIDEO Converting Between Grams and Atoms Demonstrated Example 1: If you have 12g of CH4 how many atoms of hydrogen do you have? You will need the periodic table for this question.
Step 1:
What information does the problem give you?
Answer: 12g CH4
Step 2:
What units does the question ask for?
Answer: ? atoms H
Step 3:
How many conversions must we do?
Answer: Look at the conversion map. We pass through 3 arrows when we go from Grams —> Moles —> Molecules —> Atoms. 3 arrows = 3 conversion
Step 4:
How do we set up the problem?
Answer: First box is info given, next 3 boxes are the 3 conversion, last box (fifth box) is what the question asked for.
| 12 g CH4 | atoms H |
| 1 |
Step 5:
What is the first conversion?
Answer: molar mass (grams to mole ratio) of CH4found on the periodic table
Step 6:
What is the molar mass of CH4?
Answer: about 16 g/ 1 mol
Step 7:
How do we set that up in the conversion?
Answer: units first, set up the units that need to cancel out (in red)
| 12 g CH4 | 1 mol | atoms H |
| 16 g |
Step 8:
Grams To Atoms Conversion
What conversion should we use next?
Answer: Avogadro’s number (moles to molecules ratio) found on the conversion map. 6.022 * 1023 molecules / 1 mol
| 12 g CH4 | 1 mol | 6.022 * 1023 molec | atoms H |
| 16 g | 1 mol |
Step 9:
What conversion should I use next?
Answer: the atom to molecule ratio (4 H to 1 CH4)
| 12 gCH4 | 1 mol | 6.022 * 1023molec | 4 atoms H = | atoms H |
| 16 g | 1 mol | 1 molec CH4 |
Step 10:
How do I know when I am done with the conversions?
Answer: All other units but the ones in the answer are crossed out through cancellation. For this example atoms and H remain not crossed out.
| 12 gCH4 | 1 mol | 6.022 * 1023molec | 4 atoms H = | atoms H |
| 16 g | 1 mol | 1 molecCH4 |
Step 11: Simplify
| 12 | 1 | 6.022 * 1023 | 4 atoms H = | atoms H |
| 16 | 1 | 1 |
Step 12:
How do I calculate?
Answer: (12 * 6.022 * 1023 *4) / (16) = 1.81 * 1024
Step 13:
COMPLETE ANSWER: 1.81 * 1024 atoms of H
VIDEO Converting Between Grams and Atoms Demonstrated Example 2: 6.4 * 1025 atoms of Fe is how many grams of Fe? You will need the periodic table for this question.
UNFORTUNATELY I AM NOT ABLE TO SHOW THIS DEMONSTRATED EXAMPLE ABOVE IN TEXT BECUASE THERE IS NOT ENOUGH ROOM ON THE WEBPAGE.
PRACTICE PROBLEMS: Complete grams and atoms conversions. Make sure you have this periodic table link open when answering these questions and use the conversion map if you need it.
How many F atoms are in 20g of CaF2?
Answer: 3.08 * 1023 atoms F
If you have 62 grams of Chromium how many atoms is that?
Answer: 7.18 * 1023 atoms Cr
If you have 7.89 * 1025 atoms of Boron how many grams is that?
Answer: 1.415 * 103 g B …or… 1415 g B
Grams To Atoms Conversion Formula
How many grams of Li2S can you make from 1.3 * 1024 atoms of Li?
Answer: 49.6 g Li2S
